Materials for catalysis
Mechanism and potential application of Catalysis on Metallic Alloys and Compounds
Catalysis
Identifying the role of intermetallic compounds in heterogeneous catalysis
The rich structural chemistry of intermetallic compounds allows providing peculiar combinations of the crystal and electronic structure for heterogeneous catalysis in the gas and liquid phase as well as electrocatalysis. Electronic and geometrical influences of catalysed reactions can thus be probed in a quantitative way by using electronically similar compounds or isostructural compounds with different valence electron concentration (Fig. 1).[1] Since this requires knowledge of changes to the materials under reaction conditions, research within the RAD comprises operando experiments, i.e. characterisation of the materials under catalytic conditions, catalytic testing in various reactions as well as quantum chemical calculations to enhance the understanding of the experimental results.
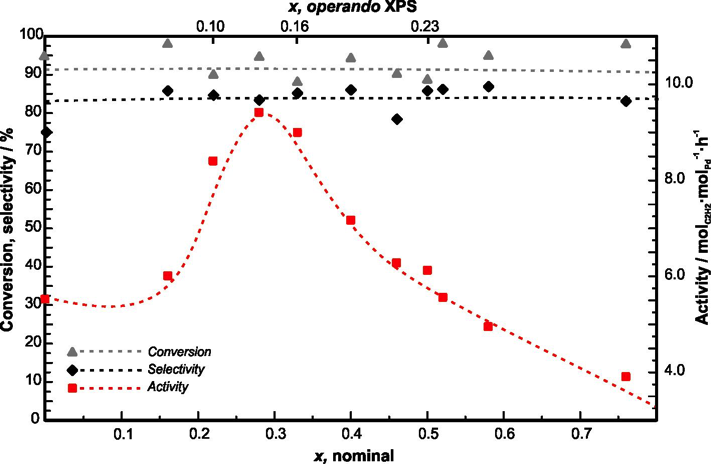
Figure 1: Catalytic properties of Ga1−xSnxPd2 in the semihydrogenation of acetylene. Conversion, selectivity to ethylene and activity of the samples are shown over the nominal composition and the composition of the near-surface region under reaction conditions as determined by XPS. Dashed lines are a guide to the eye.[1]
Besides the direct use of intermetallic compounds, they can also be used as precursors. The targeted decomposition, e.g. by controlled oxidation, can lead to materials with high specific surface area as well as a high concentration of metal-oxide interface. The latter has been proven in the last years to be of outmost importance to achieve excellent CO2-selectivity in the steam reforming of methanol (Fig. 2)[2] – an important step for the CO2-neutral Methanol Economy [3].
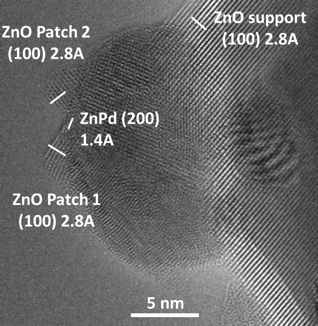
Fig. 2: HRTEM image of a single ZnPd particle (state II) with two ZnO patches on the surface, both exhibiting ZnO(100) lattice fringes of 2.8 Å. The ZnO support is also clearly visible with lattice spacings of 2.8 Å. The ZnPd lattice distances (measured at 1.4 Å) can be assigned to the (200) lattice planes.[2]
The RAD catalysis is strongly connected to the RADs Complexity and Development of new metallic alloys and compounds within the network to explore the catalytic properties of intermetallic compounds for known and new catalytic reactions as well as to develop innovative materials based on the gained knowledge.
The projects in the RAD are focussing on the following topics:
- Enhancing selectivity
-
- Selective gas-phase hydrogenation (e.g. semi-hydrogenation of acetylene)[4,5,6]
- Methanol steam reforming with exceptional CO2-selectivity[7]
- Materials with high specific surface
-
- Partial oxidation of ethylene to ethylene oxide[8]
- Development of innovative synthesis routes for supported materials[9]
- Innovative materials for electrocatalysis[10]
-
- Methanol oxidation
- Hydrogen evolution
- Direct methanol fuel cell
Results from the RAD catalysis are frequently published in highly ranked journals, presented at the ECMetAC Days and the international symposium “Intermetallic Compounds in Catalysis (IMCAT)” which is organised in the framework of ECMetAC since 2010. Recently a Focus Issue on intermetallic compounds in catalysis was published in Science and Technology of Advanced Materials (add as link https://www.tandfonline.com/toc/tsta20/20/iMC).
[1] O. Matselko, R.R. Zimmermann, A. Ormeci, U. Burkhardt, R. Gladyshevskii, Yu. Grin, M. Armbrüster, Revealing Electronic Influences in the Semihydrogenation of Acetylene, J. Phys. Chem. C 122, 2018, 21891-21896.
[2] M. Friedrich, S. Penner, M. Heggen, M. Armbrüster, High CO2 Selectivity in Methanol Steam Reforming through ZnPd/ZnO Teamwork, Angew. Chem. Int. Ed. 52, 2013, 4389-4392.
[3] G.A. Olah, G.K.S. Prakash, A. Goeppert, Anthropogenic Chemical Carbon Cycle for a Sustainable Future. J. Am. Chem. Soc. 133, 2011, 12881-12898.
[4] M. Meier, J. Ledieu, V. Fournée, É. Gaudry, Semihydrogenation of Acetylene on Al5Co2 Surfaces, J. Phys. Chem. C 121, 2017, 4958-4969.
[5] J. Prinz, O. Gröning, H. Brune, R. Widmer, Highly Enantioselective Adsorption of Small Prochiral Molecules on a Chiral Intermetallic Compound, Angew. Chem. Int. Ed. 54, 2015, 3902-3906.
[6] M. Armbrüster, K. Kovnir, M. Friedrich, D. Teschner, G. Wowsnick, M. Hahne, P. Gille, L. Szentmiklósi, M. Feuerbacher, M. Heggen, F. Girgsdies, D. Rosenthal, R. Schlögl, Yu. Grin, Al13Fe4 as a Low-Cost Alternative for Palladium in Heterogeneous Hydrogenation, Nat. Mater. 11, 2012, 690-693.
[7] K. Ploner, L. Schlicker, A. Gili, A. Gurlo, A. Doran, L. Zhang, M. Armbrüster, D. Obendorf, J. Bernardi, B. Klötzer, S. Penner, Reactive Metal-Support Interaction in the Cu-In2O3 System: Intermetallic Compound Formation and its Consequences for CO2-Selective Methanol Steam Reforming, Sci. Technol. Adv. Mater. 20, 2019, 356-366.
[8] I. Antonyshyn, O. Sichevych, K. Rasim, A. Ormeci, U. Burkhardt, S. Titlbach, S.A. Schunk, M. Armbrüster, Yu. Grin, Anisotropic Reactivity of CaAg under Ethylene Epoxidation Conditions, Inorg. Chem. 2018, 10821-10831.
[9] C. Ziegler, S. Klosz, L. Borchardt, K. Oschatz, S. Kaskel, M. Friedrich, R. Kriegel, T. Keilhauer, M. Armbrüster, A. Eychmüller, ZnPd/ZnO Aerogels as Potential Catalytic Materials, Adv. Funct. Mater. 26, 2016, 1014-1020.
[10] L. Rößner, M. Armbrüster, Electrochemical Energy Conversion on Intermetallic Compounds – A Review, ACS Catal. 9, 2019, 2018-2062.
RAD leader: I. Anthonyshyn
