Suppression of vacancies boosts thermoelectric performance in type-I clathrates
- 29
- Jan
- 2018
Xinlin Yan1, Matthias Ikeda1, Long Zhang2, Ernst Bauer1,5, Peter Rogl3, Gerald Giester4, Andrey Prokofiev1, and Silke Paschen1,5
1 Institute of Solid State Physics, Vienna University of Technology, Wiedner Hauptstr. 8–10, 1040 Vienna, Austria
2 State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, China
3 Institute of Materials Chemistry and Research, Vienna University, Währingerstr. 42, 1090 Vienna, Austria
4 Institute of Mineralogy and Crystallography, University of Vienna, Althanstr. 14, 1090 Vienna, Austria
5 Christian Doppler Laboratory for Thermoelectricity, TU Wien, Wiedner Hauptstr. 8–10, 1040 Vienna, Austria
Intermetallic type-I clathrates continue to attract attention as promising thermoelectric (TE) materials, which transfer waste heat into useful electricity and thus could be a solution for the worldwide energy crisis. The unique caged structural characteristic and the capability to accommodate different types of atoms in the framework make the type-I clathrates very interesting for both fundamental and application-oriented studies. The main obstacle to realize TE applications comes from the low charge carrier mobility, which leads to high electrical resistivity and a low energy conversion efficiency.
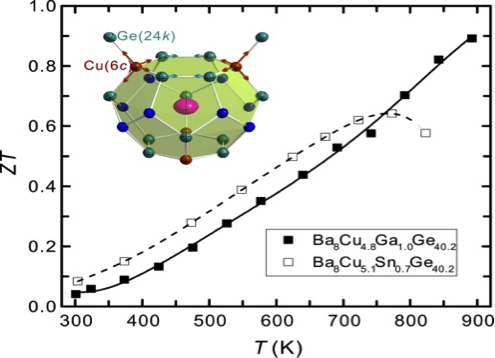
In this work, we present composition-dependent structural and thermoelectric properties of single crystalline Ba8(Cu,Ga,Ge,□)46, where □ denotes a vacancy. By single crystal X-ray diffraction on crystals without Ga, we find clear evidence for the presence of vacancies at the 6c site in the structure. With increasing Ga content, vacancies are successively filled. This increases the charge carrier mobility strongly, even within a small range of Ga substitution, leading to reduced electrical resistivity and enhanced thermoelectric performance. The largest figure of merit ZT = 0.9 at 900 K is found for a single crystal of approximate composition Ba8Cu4.6Ga1.0Ge40.4. This value, which may further increase at higher temperatures, is one of the largest found to date in transition metal element-based clathrates.